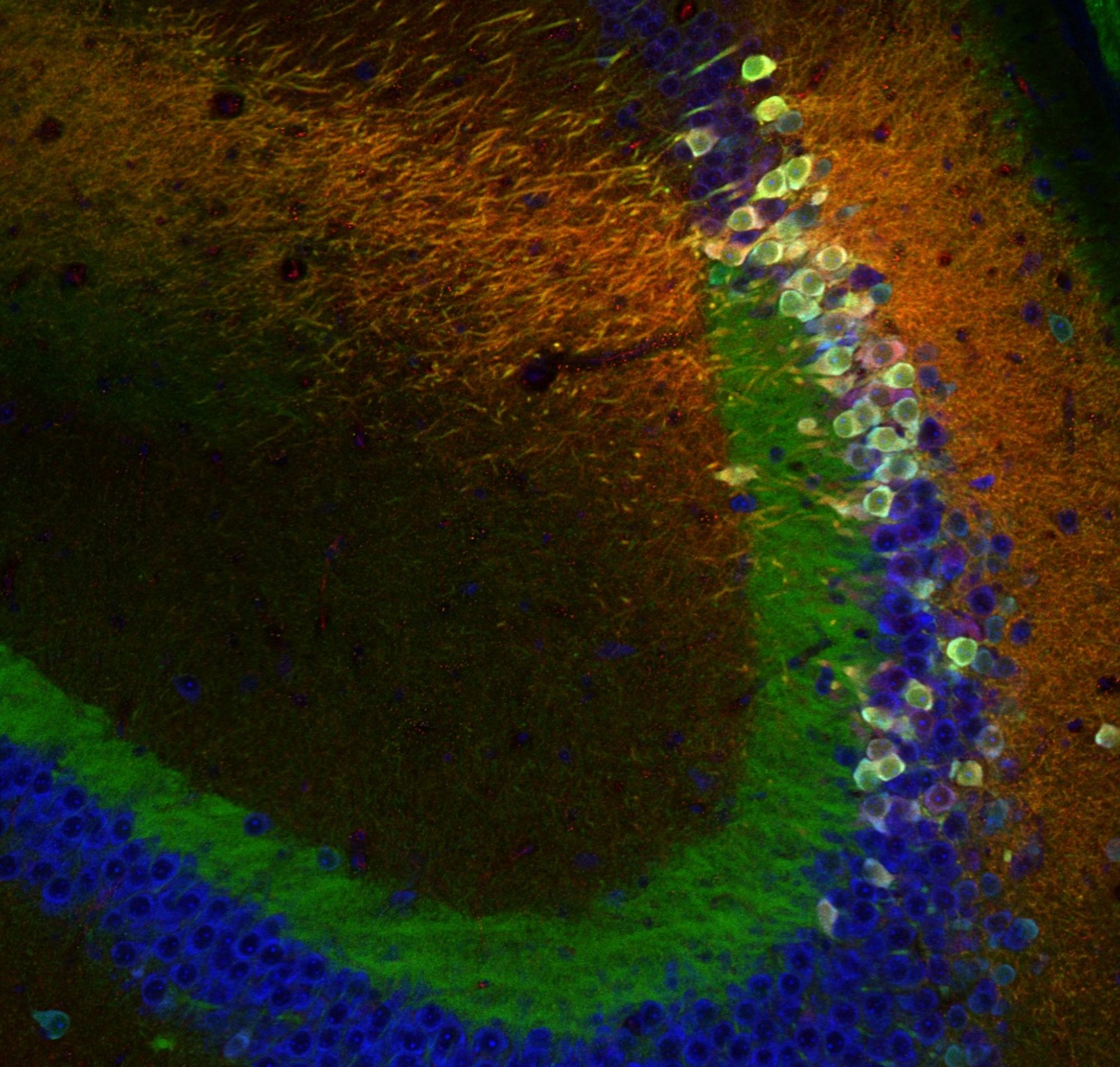
As we have been cruelly reminded of during the pandemic, social interactions are an essential part of our lives. Rodents also need social interactions and can organize in complex societies, making them a valid model to study the neural circuits supporting social interactions. For many years, the daunting task of controlling the parameters of social interaction in a laboratory setting has slowed down social behavioral research but recent technological developments (wireless in vivo recordings, tracking algorithms) have enabled it to become a hot topic in neuroscience.
We propose a 2-days scientific seminar focusing on social behavior research in rodents. This seminar will be held at the Neuroscience Institute of Alicante the 7 and 8th of October 2021. We have assembled an international roster of experts investigating different aspects of rodent social behaviors. Leader in the field will give 30 min talks to present their research and a poster session will be held to allow students to present their work and interact with principal investigators. Travel grant awardees will also present their work during a blitz oral session. The talks will be broadcasted live. Registration in person is closed but you can still register to access the talks online. Presentations will available for one month on the Youtube channel of the instituto de neurociencias.
Any inquiries should be directed to social2021@umh.es
Organizing committee
Sponsors





Scientific Program
Day 1: Thursday October 7th
| 9:30 – 10:10 | Nicolas Renier: Peptidergic neurons of the midbrain enable maternal nesting |
| 10:10– 10:50 | Susana Lima: Neural mechanisms controlling sexual behavior |
| 10:50- 11:20 | Coffee break |
| 11:20-12h | Blitz presentations |
| 12-13h30 | Posters |
| 13:30-15:30 | Lunch |
| 15:30-16:10 | Ann Clemens: Neural circuits of kinship behaviour |
| 16:10-17 | Jose-Luis Trejo: Dominating old or new friends |
| 17-17:30 | Azahara Oliva: Hippocampal mechanisms of social memory |
Day 2: Friday October 8th
| 9:30 – 10:10 | Inbal Ben-Ami Bartal: Neural circuits associated with socially selective helping behavior in rats |
| 10:10 – 10:50 | Ewelina Knapska: Second-hand emotions. What can rodents learn about the world from other’s bliss and fear? |
| 10:50-11:20 | Coffee break |
| 11:20:12h | Camilla Bellone: Neuronal circuits and synaptic mechanisms underlying social approach and avoidance behavior |
| 12-12:40 | Alexandre Charlet: Oxytocin closed-loop to promote social motivation |
| 12:40-12:45 | Concluding remarks |
Speakers

Dr. Camila Bellone, University of Geneva (Switzerland)
The laboratory of Dr. Bellone uses in vivo and ex vivo electrophysiology, behavioral paradigms and circuit manipulations, to understand the neural circuit underlying social motivation. Focusing on the meso-cortico-limbic system, we found that the activity of dopamine (DA) neurons of the Ventral Tegmental Area (VTA) encodes social prediction error. Mutations in ASD-related genes in animal models, alters the activity of VTA neurons and causes deficits in social motivation.
- Dopamine neurons of the VTA encode active conspecific interaction and promote social learning through social reward prediction error. Clément Prévost-Solié, Benoit Girard, Beatrice Righetti, Malika Tapparel, Camilla Bellone. bioRxiv 2020.05.27.118851
- Superior Colliculus to VTA pathway controls orienting response to conspecific stimuli. Clément Prévost-Solié, Alessandro Contestabile, Pedro Espinosa, Stefano Musardo, Sebastiano Bariselli, Chieko Huber, Alan Carleton, Camilla Bellone. bioRxiv 735340.
- Role of VTA dopamine neurons and neuroligin 3 in sociability traits related to nonfamiliar conspecific interaction. Bariselli S, Hörnberg H, Prévost-Solié C, Musardo S, Hatstatt-Burklé L, Scheiffele P, Bellone C. Nat Commun. 2018 Aug 9;9(1):3173.
- SHANK3 controls maturation of social reward circuits in the VTA. Bariselli S, Tzanoulinou S, Glangetas C, Prévost-Solié C, Pucci L, Viguié J, Bezzi P, O’Connor EC, Georges F, Lüscher C, Bellone C. Nat Neurosci. 2016 Jul;19(7):926-934.

Inbal Ben-Ami Bartal, Tel -Aviv University (Israel)
The Ben-Ami Bartal laboratory researches the neurological basis of prosocial behavior, focusing on a rodent model of helping, where rats can help another rat by setting it free from a trap. Her lab employs molecular and computational tools to study this issue from the cell level, through neural networks, to behavior. Her research focuses on understanding how the brain processes the distress and pain of others, why we experience empathy for some but not other individuals or groups, and how the decision to help others in need is computed by the brain. Using an integrative approach that combines behavior, immunostaining and optogenetic tools, the lab examines the brain’s response to a trapped animal and during the act of helping, in order to outline the molecular pathway involved in prosocial approach. This research seeks to inform our basic understanding of the mechanism underlying social behavior and provides a translational path for developing novel therapeutic approaches for deficits in social behavior stemming from psychopathology or trauma.
- Pro-social behavior in rats is modulated by social experience. Ben-Ami Bartal I, Rodgers DA, Bernardez Sarria MS, Decety J, Mason P. Elife. 2014;3:e01385. doi: 10.7554/eLife.01385. Epub 2014 Jan 14. PMID: 24424411; PMCID: PMC3884117.
- Empathy and pro-social behavior in rats. Ben-Ami Bartal I, Decety J, Mason P. Science. 2011 Dec 9;334(6061):1427-30. doi: 10.1126/science.1210789. Erratum in: Science. 2012 Jan 27;335(6067):401. PMID: 22158823; PMCID: PMC3760221.
- Empathy and pro-social behavior in rats. Ben-Ami Bartal I, Decety J, Mason P. Science. 2011 Dec 9;334(6061):1427-30. doi: 10.1126/science.1210789. Erratum in: Science. 2012 Jan 27;335(6067):401. PMID: 22158823; PMCID: PMC3760221.

Alexandre Charlet, University of Strasbourg (France)
The Charlet lab is interested in the oxytocinergic modulation of neuronal circuits underlying emotions, with a special focus in the central amygdala. Using ex vivo patch-clamp and calcium imaging combined with selective optogenetic manipulation of oxytocinergic neurons or local astrocytes, we currently examine how oxytocin can modulate the amygdala neuro-glia network involved in the emotional process such as pain and anxiety.
- Astrocytes mediate the effect of oxytocin in the central amygdala on neuronal activity and affective states in rodents. Wahis J, Baudon A, Althammer F, Kerspern D, Goyon S, Hagiwara D, Lefevre A, Barteczko L, Boury-Jamot B, Bellanger B, Abatis M, Da Silva Gouveia M, Benusiglio D, Eliava M, Rozov A, Weinsanto I, Knobloch-Bollmann HS, Kirchner MK, Roy RK, Wang H, Pertin M, Inquimbert P, Pitzer C, Siemens J, Goumon Y, Boutrel B, Lamy CM, Decosterd I, Chatton JY, Rouach N, Young WS, Stern JE, Poisbeau P, Stoop R, Darbon P, Grinevich V, Charlet A. Nat Neurosci. 2021 Apr;24(4):529-541.
- Social touch promotes interfemale communication via activation of parvocellular oxytocin neurons. Tang Y, Benusiglio D, Lefevre A, Hilfiger L, Althammer F, Bludau A, Hagiwara D, Baudon A, Darbon P, Schimmer J, Kirchner MK, Roy RK, Wang S, Eliava M, Wagner S, Oberhuber M, Conzelmann KK, Schwarz M, Stern JE, Leng G, Neumann ID, Charlet A, Grinevich V. Nat Neurosci. 2020 Sep;23(9):1125-1137.
- A Fear Memory Engram and Its Plasticity in the Hypothalamic Oxytocin System. Hasan MT, Althammer F, Silva da Gouveia M, Goyon S, Eliava M, Lefevre A, Kerspern D, Schimmer J, Raftogianni A, Wahis J, Knobloch-Bollmann HS, Tang Y, Liu X, Jain A, Chavant V, Goumon Y, Weislogel JM, Hurlemann R, Herpertz SC, Pitzer C, Darbon P, Dogbevia GK, Bertocchi I, Larkum ME, Sprengel R, Bading H, Charlet A, Grinevich V. Neuron. 2019 Jul 3;103(1):133-146.e8.

Dr. Ann Clemens, University of Edinburgh (Scotland)
Dr. Clemens is investigating neuronal circuits regulating kinship behavior. “Our work is focused on understanding the sensory and circuit mechanisms underlying social behavior from an ethological perspective. We examine the stages of social-sensory processing beginning with recognition and ending with social attachment. In between, we hope to disentangle both innate and learned aspects of kinship social behavior by probing and observing the dynamics of the underlying neural circuits.”
- The lateral septum mediates kinship behavior in the rat. AM Clemens, H Wang, M Brecht. (2020) Nature communications 11 (1), 1-11
- Estrus-cycle regulation of cortical inhibition. Ann M Clemens, Constanze Lenschow, Prateep Beed, Lanxiang Li, Rosanna Sammons, Robert K Naumann, Hong Wang, Dietmar Schmitz, Michael Brecht. (2018) Current Biology 29 (4), 605-615. E6

Dr. Ewelina Knapska, Nenki Insitute (Poland)
Dr. Knapska leads the Laboratory of Emotions Neurobiology, at the Nencki Institute of Experimental Biology, Warsaw, Poland. Her laboratory focuses on understanding how the brain controls social life is one of the most fascinating quests of neuroscience. Although social interactions among individuals are central to most animals, including humans, we are only beginning to understand the underlying neural processes. In our laboratory we study mechanisms of social interactions at the level of neural circuits and cells using simple animal models. We combine rat and mouse behavioral models with optogenetic, chemogenetic, electrophysiological, and imaging techniques. The two main questions our research is focused on are: (1) Are the neural circuits underlying positive and negative social emotions distinct? (2) Are there neural circuits specialized in social emotions?
Malfunctioning neural circuits governing social interactions are responsible for a number of neuropsychiatric disorders, including autism spectrum disorder and psychopathy. Our research focuses on the brain circuits and cellular mechanisms underlying impaired social interactions. We are trying to test the possibilities for therapeutic intervention in mouse genetic and idiopathic models of autism spectrum disorder. Using state-of-the-art automatic systems for assessing social behavior and neurobiological tools we would like to explain why some individuals are more likely to suffer from autism than others.
- Chronic fluoxetine treatment impairs motivation and reward learning by affecting neuronal plasticity in the central amygdala. Puścian A, Winiarski M, Łęski S, Charzewski Ł, Nikolaev T, Borowska J, Dzik JM, Bijata M, Lipp HP, Dziembowska M, Knapska E. (2021) Br J Pharmacol. 178(3): 672-688.
- The neural and computational systems of social learning. Olsson A, Knapska E, Lindström B. (2020) Nat. Rev. Neurosci. 21(4): 197-212.
- The roots of empathy: Through the lens of rodent models. Meyza KZ, Bartal IB, Monfils MH, Panksepp JB, Knapska E. (2017) Neurosci. Biobehav. Rev. 76(Pt B): 216-234.
- Eco-HAB as a fully automated and ecologically relevant assessment of social impairments in mouse models of autism. Puscian A, Leski S, Kasprowicz G, Winiarski M, Borowska J, Nikolaev T, Boguszewski PM, Lipp HP, Knapska E. (2016) Elife. 5:e19532.

Dr. Susana Lima, Champalimaud Center for the Unknown (Portugal)
The main goal of Dr. Lima’s laboratory is to gain mechanistic insights into the neuronal processes underlying sexual behavior, in particular its initiation (modulation of female sexual behavior by sex hormones) and its termination (ejaculation and the refractory period in males). To do so, we use mice as model system and a combination of approaches that include physiological, anatomical and molecular tools to dissect the contribution of candidate brain areas to the emergence of these natural behaviors.
- The structural and electrophysiological properties of progesterone receptor expressing neurons vary along the anterior-posterior axis of the ventromedial hypothalamus and undergo local changes across the reproductive cycle. Ines C Dias, Nicolas Gutierrez-Castellanos, Liliana Ferreira, Susana Q Lima. BiorXiv doi: https://doi.org/10.1101/2021.02.02.429415 (under review)
- Valente, S, Marques, T and Lima, SQ. No evidence for prolactin’s involvement in the post-ejaculatory refractory period. Commun Biol 4, 10 (2021).
DOI: https://doi.org/10.1038/s42003-020-01570-4 - Moreira L, Zinck L, Nomoto K and Lima SQ. (2020). Sexual imprinting overrides order effects during sampling of prospective mates. Current Biology, March 2020 DOI: https://doi.org/10.1016/j.cub.2020.02.033
- Lenschow C and Lima SQ. (2019). In the Mood for Sex: Neural Circuits for Reproduction. Curr Opin Neurobiology, 60, 155-168 Feb 2020
PMID: 31901622 DOI: 10.1016/j.conb.2019.12.001

Dr. Azahara Oliva, Cornell University (USA)
Although the hippocampus is widely acknowledged for its role in spatial navigation, this region is involved in much more complex forms of declarative memory, including social memory. The consolidation of spatial memory requires the reactivation of previously active place cells during sharp-wave ripples (SWRs), hippocampal oscillations that occur during non-REM sleep. The underlying substrate of other types of memory, such as social memory, were poorly understood. Dr Oliva uses in-vivo electrophysiology combined with optogenetics to describe the hippocampal mechanisms for encoding and consolidating the memory of a conspecific. She found that single cells in the hippocampus remap with the location of conspecifics and these cells are later reactivated during SWRs. Social memory was impaired by closed-loop disruption of SWRs and prolonged by artificial generation of SWRs.
- Parallel pathways for mnemonic processing. Oliva A. (2020) Trends in Neuroscience 44(2):79-81
- Selective gamma rhythm communication between entorhinal cortex and dentate gyrus neuronal assemblies supports learning. Fernández-Ruiz A, Oliva A, Soula M, Rocha-Almeida F, Nagy GA, Martin-Vazquez G, Buzsaki G. (2020) Science, In press.
- Hippocampal CA2 ripples reactivate and promote social memory. Oliva A, Fernández-Ruiz A, Leroy F, Siegelbaum S. (2020) Nature, 587, 264-269
- Long-duration Hippocampal Sharp Wave Ripples Improve Memory. Fernández-Ruiz A, Oliva A, Fermino de Oliveira E, Rocha-Almeida F, Tingley D, Buzsáki G. (2019) Science 364(6445),1082-1086.
- Oliva A, Fernández-Ruiz A, Buzsáki G, Berényi A. (2016) Role of hippocampal CA2 region in triggering sharp-wave ripples. Neuron 91,1342-1355.

Dr. Nicolas Renier, Brain and Spine Institute (France)
Dr. Renier is known for developing new clearing techniques to image the whole brain (iDISCO), including for c-fos expression. He is currently applying these tools to investigate how a small hypothalamic nucleus can regulate maternal nests.
Optimizing reproductive fitness in mammals requires a series of behavioral adaptations during pregnancy such as rest, nesting, and feeding. We are looking to identify key neurons modulating the balance between behavioral states during early gestation. For this, we are screening for brain-wide immediate early gene activation with a focus on nesting behavior. Based on this screen, we are now focusing our efforts on understanding the role on parental behaviors of an elusive population of peptidergic midbrain neurons located in the Edinger-Westphal region.
- iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M. Cell. 2014 Nov 6;159(4):896-910. doi: 10.1016/j.cell.2014.10.010. Epub 2014 Oct 30.
- Mapping of Brain Activity by Automated Volume Analysis of Immediate Early Genes. Renier N, Adams EL, Kirst C, Wu Z, Azevedo R, Kohl J, Autry AE, Kadiri L, Umadevi Venkataraju K, Zhou Y, Wang VX, Tang CY, Olsen O, Dulac C, Osten P, Tessier-Lavigne M. Cell. 2016 Jun 16;165(7):1789-1802. doi: 10.1016/j.cell.2016.05.007. Epub 2016 May 26.

Dr. Jose-Luis Trejo, Cajal Institute (Spain)
Dr. Trejo is interested in how the social dominance hierarchy progresses from an initial, acute first-encounter dominance to an established, chronic social hierarchy. Our analyses focus on how lifestyle (physical exercise and stress) influences social motivation and hierarchy, and our approach is focused on hippocampal CA2, mPFC and paraventricular nucleus, by analyzing gene expression, adult hippocampal neurogenesis, vomeronasal receptors, and hierarchy-specific urine effects.
- Social dominance differentially alters gene expression in the medial prefrontal cortex without affecting adult hippocampal neurogenesis or stress and anxiety-like behavior. Pallé A, Zorzo C, Luskey VE, McGreevy KR, Fernández S, Trejo JL. FASEB J. 2019 Jun;33(6):6995-7008. doi: 10.1096/fj.201801600R. Epub 2019 Mar 11. PMID: 30857420.
- Gαi2+ vomeronasal neurons govern the initial outcome of an acute social competition. Pallé A, Montero M, Fernández S, Tezanos P, de Las Heras JA, Luskey V, Birnbaumer L, Zufall F, Chamero P, Trejo JL. Sci Rep. 2020 Jan 21;10(1):894. doi: 10.1038/s41598-020-57765-6. PMID: 31965032; PMCID: PMC6972791.